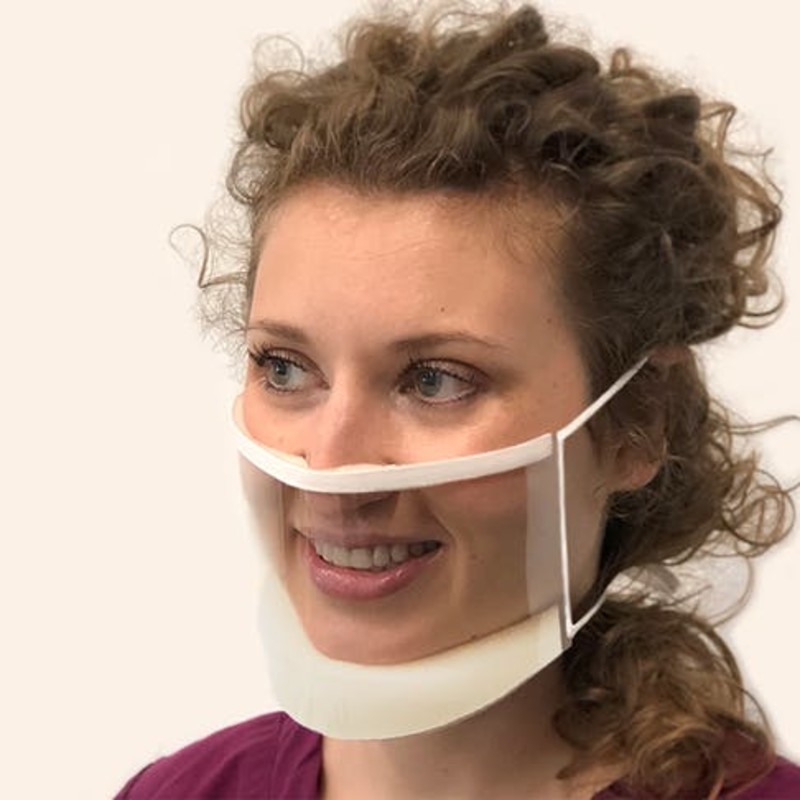
In addition to facilitating the production of many different types of PPE, Maryland-based Xometry’s on-demand manufacturing marketplace was critical in enabling the production of an innovative transparent mask, ClearMask™, now available in both medical and non-medical versions in the marketplace at https://buy.theclearmask.com/.
Developed by ClearMask LLC, a Maryland-based medical company, the mask is a fully transparent, FDA-approved surgical mask that is essential for medical personnel and doctors who are deaf or hard of hearing and rely on facial expressions, visual cues, and lipreading to communicate. ClearMask blocks infectious particles and droplets through its transparent plastic barrier and has an anti-fog coating.
“ClearMask was approved by the FDA in April 2020, but the company was challenged with finding a manufacturer capable of producing and shipping very high volumes of the masks weekly,” said Greg Paulsen, director of application engineering, Xometry. “We identified a company in our global network of 5,000+ manufacturing suppliers who could produce and assemble millions of masks rapidly and cost effectively. We went beyond the role of supply chain partner and also served as a logistics expert and engineering consultant on this important project.”
Xometry is still managing and coordinating production between the ClearMask team and the supplying facility to ensure production goals are met. Millions of masks have been produced for medical personnel as well as state emergency management agencies, hospitals, essential workers, and different communities in need.
In addition to supporting those who work with deaf and hard of hearing individuals (worldwide there are more than 455 million people who are deaf), ClearMask is important for anyone who benefit from visual facial cues and full facial expressions. Apple, for example, has approved two masks for employee use. One mask is designed by Apple, and the other is ClearMask.